Chemical Education in Asia-Pacific
CEMICAL EDUCATION IN HONG KONG
Lo Mun Ling
Department of Curriculum Studies, The University of Hong
Kong, Pokfulam Road, Hong Kong
Back to Chemical
Education in Ajia Pacific Home Page
1. THE EDUCATIONAL SYSTEM
OF HONG KONG
In Hong Kong, education is highly valued by most parents, as is expected
of the Chinese culture. The basic structure of schooling is that most children
attend Kindergartens from the age of 3 to 6. This is followed by 6 years
of primary schools and then 7 years of secondary schools which is split
into three programmes; junior secondary S1 - S3, senior secondary S4 -S5
and S6 -S7. Nine years of compulsory education was introduced in 1971.
Public sector places are free of tuition fees for children between the
age of six to fifteen (or up to the completion of Secondary 3, whichever
is earlier ). Schools are allowed to charge a small amount of 'Tong Fai'
(subscriptions) which may be up to a few hundred dollars per year and varies
from school to school. Despite the fact that enough places are available
in the public sector, about 10% of students are still studying in fee paying
private schools as a result of parental choice. About 85% of students can
continue onto upper secondary 4 and 5 in highly subsidized places. At the
end of Secondary 5, students have to take the Hong Kong Certificate of
Education Examination. About 30% can then continue to Secondary 6 and 7
leading to the Hong Kong Advanced Level Examination. Of these, 24% can
go on to public-sector tertiary education courses at degree or sub-degree
level.
The official version of the fundamental aim of school education in Hong
Kong is to -
"develop the potential of every individual child, so that our
students become independent-minded and socially-aware adults, equipped
with the knowledge, skills and attitudes which help them to lead a full
life as individuals and play a positive role in the life of the community."
(A guide to Education and Training in Hong Kong, Education & Manpower
Branch, Government Secretariat, Dec. 1994. )
Top of Page
2. A GENERAL SURVEY OF CHEMISTRY
EDUCATION
Chemistry is not a formal component of the primary curriculum. The only
topics that may have some components of chemistry in them are "air"
and 'water' in the General Studies curriculum which was created in 1985
as an integration of the three existing subjects, social studies, health
education and science. For most students, their first contact with chemistry
is in the junior secondary curriculum when they study Integrated Science
which is an integration of the contents of the three subjects Chemistry,
Physics and Biology. The normal practice is to have 4 periods of Integrated
Science per five-day-week or 5 periods per six-day-cycle. The Integrated
Science curriculum is intended for Secondary 1 to 3. However, in Secondary
3, many schools choose to begin preparing students for the Hong Kong School
Certificate Examination, and the science program is split into Biology,
Chemistry and Physics, taught by three separate teachers, each taking up
two periods.
At Secondary 4 and 5, students may opt for an arts stream or a science
stream. Chemistry will not be taken by students studying an arts stream.
The basic pattern of subjects followed by a science student in secondary
schools is shown in Fig. 1. It can be seen
that in the science stream, it is common to have 4 periods of Chemistry
per five-day-week or 5 per six-day-cycle.
Prior to 1991, it was common for Secondary 6 students to take The Use
of English and three A-level subjects. For the science stream, these may
be any three subjects out of the group Pure Mathematics, Applied Mathematics,
Chemistry, Physics or Biology. The more able students may take up to 4
A-level subjects. A-level Computer Studies was only introduced in 1991.
From 1992, following the recommendation of the Education Commission1 Report
No. 2 to broaden the Sixth Form curriculum and to improve communication
skills, students in Secondary 6 have to take both English and Chinese and
they are encouraged to take a combination of A-level and AS-level subjects.
The A-level subjects are for depth while the AS level subjects are for
broadening. Schools may offer Advanced level chemistry or AS(Advanced Supplementary)
Level chemistry. An AS-level subject is counted as half an A-level subject.
The number of periods varies from 8 to 12 for A-level and 5 to 6 for AS-level.
Below, I elaborate on the chemistry curriculum at each of the levels of
schooling within Hong Kong.
| S5 |
|
|
3 or 4 subjects out of the group of subjects:
Physics
Chemistry
Biology
Applied Maths
(15) |
One humanities subject or Computer Studies
(3) |
P.E. (non-examination)
(2) |
| S4 |
|
|
| S3 |
|
|
|
|
|
|
|
| S2 |
English Language
(10) |
Chinese Language
(9) |
Maths
(9) |
Integrated Science
(5) |
Geog., EPA, History, Chinese Hist./ Social studies
(9) |
Computer Literacy
(1) |
Cultural & practical subjects: Music, P.E., D&T, Form Teacher
Period
(5) |
| S1 |
|
|
|
|
|
|
|
|
10 |
19 |
28 |
33 |
42 |
43 |
48 |
No. of periods per cycle
Fig. 1 A Curriculum map for S1 - S5, Science Stream
(Adopted from Morris, P., (1995), The Hong Kong School Curriculum,
Development, Issues and Policies, Hong Kong University Press )
Top of Page
3. THE CHEMISTRY CURRICULUM
AT JUNIOR SECONDARY LEVEL
Before 1974, education in Hong Kong was highly selective and only the
academically most able students could go on to subsidized secondary schools.
At junior secondary level, most schools followed a General Science Curriculum.
The teaching method was mainly didactic and expository, students did not
have any chance of doing experiments and even teacher demonstrations were
extremely rare. With the introduction of free and compulsory education
for all up to Secondary 3, the need to cater for a different target population
with very different and a wide range of abilities became urgent. The Scottish
Integrated Science curriculum was looked upon by the Government as the
solution. A pilot project involving 29 schools was set up to try out the
curriculum and substantial resources were poured into the project. Training
courses for teachers and educators were organized and taught by four tutors
from Scotland who had first-hand experience in the formulation and implementation
of the Integrated Science curriculum in Scotland. In Sept., 1976, adoption
was open to other schools which received support in terms of a recurrent
grant for chemicals and equipment, the creation of more technician posts,
provision of more laboratories and training courses for teachers and, ETV
programmes were produced to support the curriculum. The Hong Kong Association
for Science and Mathematics Education (HKASME) also greatly enhanced its
adoption by designing and selling home-made equipment and offering training
courses. In fact, Integrated Science is one of the few educational innovations
that have been successfully institutionalized in Hong Kong and contrasts
markedly with the experience with other integrated subjects such as social
studies and liberal studies.
The Integrated Science syllabus was divided into 15 sections as shown
in Table 1.
An experimental approach was encouraged and the stated objectives of
the course was to provide pupils with
1. some knowledge of the empirical world around him (sic)
2. a little of the vocabulary and grammar of science
3. an ability to observe objectively
4. an ability to solve problem situations and think scientifically
5. an awareness of the culture which is science
There has been very little change to the syllabus since its introduction
in 1974. The only major change is the addition of two topics: electronics
and building materials in the 80's to make the course more relevant to
the local context at a time when Hong Kong had a flourishing electronics
industry and as a 'concrete jungle'.
Table 1. 15 sections of The Integrated Science
syllabus
|
Section |
Subject matter included |
| 1 |
Introducing science |
physics, chemistry & biology |
| 2 |
Looking at living things |
biology |
| 3 |
Energy |
physics, chemistry & biology |
| 4 |
What are things made of |
physics & chemistry |
| 5 |
Solvents and solutions |
chemistry & biology |
| 6 |
Cells and reproduction |
biology |
| 7 |
Electricity |
physics |
| 8 |
Some common gases |
chemistry & biology |
| 9 |
Making heat flow |
physics & biology |
| 10 |
Hydrogen, acids and alkalis |
chemistry |
| 11 |
Detecting the environment |
biology & physics |
| 12 |
The Earth and what we get from it |
chemistry & biology |
| 13 |
Support and movement |
biology & physics |
| 14 |
Transport systems in living things |
biology |
| 15 |
Electricity and magnesium |
physics |
During the initial implementation, there was no suitable text books
for teachers to follow. The only major printed resource material available
was the Heinemann Worksheets adopted for Hong Kong. Consequently, teachers
were forced to create their own teaching materials and there were tremendous
professional activities during those years leading to professional growth
for many science teachers. However, later, efforts turned to the writing
of very detailed curriculum guides and the production of ETV to support
teaching. This was perhaps well meaning because these provided useful guidance
to the novice teacher, however, without further training and professional
exchange, new teachers coming onto the scene perceive these curriculum
guides as prescriptive.
A small section of the curriculum guide is included here:
=========================================================================
C.4 particles mixing and spacing
Expected outcome
Pupils should be able to infer that there are spaces between the particles
|
Activities |
Notes |
| 1. |
Mix equal known volumes of alcohol and water. measure the total volume
and compare the result with the sum of the two volumes. |
When equal volumes of alcohol (ethanol) and water are mixed, the total
volume is less than the sum of the two volumes. |
|
Suggest explanation for any difference in the total volume. |
A possible explanation of this is that both liquids consist of particles
and that the particles of one can slip into the spaces between the particles
of the other. |
| 2. |
Mix equal know volumes of peas and rice. Measure the total volume and compare
the result with the sum of the two volumes. |
This helps to consolidate the idea that there are spaces between the particles. |
======================================================================
Text books used by schools in Hong Kong have to go through a vigorous
reviewing process by the Curriculum Development Institute. Only text books
in the recommended book list can be adopted by schools. Thus, text book
writers do not have much freedom with the approaches and choice of materials
since the content is already defined by the syllabus and the curriculum
guides define in minute details the approaches. As a result, although there
are many sets of Integrated Science text books by various publishers, they
all look very similar in content and approaches. Most text books are printed
on A4 size paper and in full colour. Since the official medium of instruction
in most secondary schools is English and yet many students are not proficient
enough to learn in this medium, the characteristics of text books are that
very simple English is used and many diagrams are used for illustration.
The students' text books are kept as simple as possible while most of the
details, answers, extension materials, suggestions for treatment of various
topics, teaching methodologies, etc. are put into the teachers' guides.
A page of the teachers' edition of a popular Integrated Science text
book is included below. The students' text would be identical except that
the answers and notes for the teachers would be omitted.
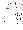 A page of a popular Integrated
Science text book
A page of a popular Integrated
Science text book
(Page taken from Lo, M.L. et al, (1992), Integrated Science, A New
Approach, Book 1A, Teachers’ edition, 3rd edition, Macmillan Publishers
(HK) Ltd.)
With a curriculum guide that is set out in such detail and text books
which are very close reflections of the curriculum guide, a 'fidelity of
use' perspective is encouraged towards implementation. Unfortunately, this
is not true in reality. Most science teachers just teach following strictly
the text book using a cook-book approach. Whereas before the introduction
of Integrated Science, students seldom had any chance to do experiments
on their own and they were thrilled at the chance of being able to get
hands on experience, now, students are bored with too much experimentation
but not knowing why they are doing experiments. The spirit of the enquiry
approach is lost. A syllabus revision for Integrated Science is now underway,
hoping to revive some of the more worthy goals of science education. Some
schools with a large intake of academically low achievers are also trying
to develop their own school-based curriculum by tailoring the present curriculum
to suit the needs of their own students. The SMILE (Science as a Motivating
and Invigorating Learning Experience) project is an attempt to change the
teaching and learning of science in Hong Kong schools. This is a project
initiated by a group of secondary school teachers and some teaching staff
of the Faculty of Education of the University of Hong Kong. At the moment,
a few pilot schools are involved. It is hoped that it might gather more
momentum later.
Top of Page
4. THE CHEMISTRY CURRICULUM
AT SENIOR SECONDARY LEVEL
Prior to 1985, there was no formal institute responsible for conducting
research in curriculum development in Hong Kong. Most curricula were direct
copies from overseas. The Integrated Science curriculum for Junior Secondary
Education was imported directly from Scotland and the Chemistry syllabus
for the School Certificate Examination was just an adaptation of the Nuffield
Chemistry Project in the United Kingdom. The Advanced level chemistry syllabus
was copied from the General Certificate of Education (GCE) Advanced Level
Chemistry Syllabus of the University of London. The situation has improved
slightly after the Curriculum Development Council was reconstituted and
upgraded in 1992 in response to the Education Commission Report No.4 (1990).
The Council is now appointed by the Governor instead of by the Director
of Education and it includes parents, employers as well as professional
educators. In 1992, a new division, the Curriculum Development Institute
was set up in the Education Department.
Of the three science subjects (Physics, Biology and Chemistry), the
chemistry curriculum seems to be the more progressive. For example, the
Teacher Assessment Scheme for A-level practical chemistry was piloted in
1973-75 and first implemented in 1977 while the TAS for biology is only
just implemented and there is still no attempt made for Physics. This scheme
places responsibility for the assessment of practical skills which include
manipulative skills, skills in planning experiments and the interpretation
of data in the hands of teachers. Continuous assessments by teachers are
used and teachers are encouraged to integrate theory and practical in their
teaching so that a truly enquiry/experimental approach can be possible.
The chemistry curriculum was also the first to respond to the Science-Technology-Society
movement and this is reflected in the new syllabus for Secondary 4 and
5, implemented in 1993 and examined for the first time in 1995. As can
be seen from the synopsis below, although for the most part, a very strong
subject discipline orientation is still maintained, an attempt has been
made to incorporate in part the STS (Science-Technology-Society
) element with an 'application-first approach'.
=========================================================================
SYNOPSIS OF SYLLABUS FOR SECONDARY 4 & 5
| Section 1. Fundamentals of matter |
Section 5. Fossil Fuels |
1.1 Elements
1.2 Atoms
(a) Structure of atoms
(b) Mass of atoms
(c) Electronic arrangement of atoms
1.3 The Periodic Table
1.4 Elements and compounds
1.5 Bonding in compounds
(a) Ionic bonding
(b) Covalent bonding
1.6 Ionic and covalent substances |
5.1 Fossil fuels
5.2 Distilled fractions from petroleum
5.3 Homologous series. Structural formulae and naming of carbon compounds
of not more than four carbon atoms
5.4 Demand for fractions from petroleum as fuels
5.5 Burning of fuels and fire fighting
5.6 Environmental problems associated with the use of fuels |
| Section 2. Common Metals |
Section 6. Important Products from Petroleum |
2.1 Uses of common metals
2.2 Reactivity of metals
2.3 Corrosion of metals and their protection
2.4 Alloys |
6.1 Plastics
6.2 Alkanols
6.3 Detergents |
| Section 3. Chemical Cells and Electrolysis |
Section 7. Important Industrial Products |
3.1 Simple chemical cells
3.2 Redox reactions
3.3 Other chemical cells
3.4 Electrolysis |
7.1 Nitrogenous fertilizers
7.2 Bleach |
| Section 4. Common Acids and Alkalis |
Section 8. Chemicals and Health |
4.1 Domestic acids and alkalis
4.2 Characteristics of acids
(a) Dilute acids
(b) Concentrated acids
4.3 Concentration of acids
4.4 Characteristics of alkalis
4.5 pH
4.6 Strength of acids and alkalis
4.7 Neutralization
4.8 Simple volumetric work involving acids and alkalis |
8.1 Food additives
8.2 Drugs |
=========================================================================
The syllabus emphasizes social relevance, especially in the areas of
environment, energy, industry and health. It is stated that chemistry and
society is the unifying theme and suggests that 'teaching goes from society
to the conceptual framework rather than from the underlying principles
to their applications' and the teaching of skills in problem-solving, enquiry,
communication and decision making is emphasized.
Although there is still a very detailed teaching syllabus published
by the Curriculum Development Council and is recommended for use in schools
by the Education Department, it is not as detailed and prescriptive as
was the case with Integrated Science.
A page of a text book is included here. Starting with the idea that
one can see the movement of ions because most ions are coloured, students
carry out experiments to find out the colour of different ions and to predict
their charges. Then the colour of gemstones as a result of the presence
of different coloured ions are discussed.
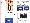 A page of a text book starting with the
idea that one can see the movement of coloured ions
A page of a text book starting with the
idea that one can see the movement of coloured ions
(Page taken from Doyle, P & Lo, M.L., (1993), Chemistry Today, Book
1, Longman Group (Far East) Ltd. )
Following the spirit of the syllabus, a small group of chemistry teachers
has been involved in the development of school based curriculum modules
based on the Science-technology-society approach. Chemistry is introduced
within these modules on a 'need to learn' basis. A few interesting modules
are described below.
1. Studying the problem of water pollution of a nearby river.
2. Starting with the idea of renovating an old school to celebrate its
80th anniversary, students investigated the use of different building materials
and their properties.
3. A school is situated near to a petrol station. Starting with the problem
of 'What happens if the petrol station is on fire?", students investigated
what is petrol, what happens when petrol burns and this later developed
into a study of fire fighting and finally resulted in a proposal for changing
the fire drill procedure for the school.
However, this should not give an illusion that the teaching of chemistry
is very progressive in Hong Kong. Such teachers only represent a minority.
Most chemistry teachers, unfortunately, are still using very traditional
expository methods which are in complete contrast to what is advocated
in the curriculum guides.
Top of Page
5. The Chemistry Curriculum at
Sixth Form Level
In the past, because of the strong competition for tertiary places,
there was very great pressure for schools to prepare students in Sixth
Form for the A-level examinations. The backwash effect went right through
to primary schools. With the expansion of tertiary education, the aim of
sixth form education was re-examined and Education Commission Report Number
2 (1986) recommended a number of objectives for sixth form education. These
were:
a. to encourage the development of balanced, well-informed individuals;
b. to prepare students for post-secondary and tertiary education;
c. to develop in all students the ability to communicate effectively
in both English and Chinese;
d. to prepare students for adult life.
A working group was set up to make recommendations on how to broaden
the curriculum for sixth form education. In 1992, following the recommendations
of the Report of the Working Group, the content of the A-level chemistry
syllabus was trimmed down by 20% to make way for more subjects to be studied
in Form Six, e.g. Liberal Studies.
In 1995, a new revised syllabus with a section on chemistry and society
was introduced and will be examined for the first time in 1997 and is shown
below. More details are included only for topic 14 since topics 1 to 13
are the traditional topics.
=========================================================================
SYNOPSIS OF THE A-LEVEL SYLLABUS
1. Atoms, Molecules and Stoichiometry
2. The Electronic Structure of Atoms and the Periodic Table
3. Energetics
4. Bonding and Structure
5. Chemical Kinetics
6. Chemical Equilibria
7. Phase Equilibrium
8. Periodic Properties of the Elements in the Periodic Table
9. The s-Block Elements
10 The p-Block Elements
11 The d-Block Elements
12. Fundamentals of Organic Chemistry
13. Chemistry of the Organic Compounds
14. Chemistry and Society
14.1 Chemistry and the environment
(a) Air pollution
(b) Water pollution
(c) Solid waste
(d) Pollution control in Hong Kong
14.2 Chemistry and food
(a) Principal components of food
(b) Food preservation
(c) Food additives
======================================================================
Prior to 1976, there was only one mode of A-level practical examination.
It consisted of two parts, a qualitative analysis and volumetric analysis.
However, the practical examination had frequently been criticized because
of the backwash effect it caused - teachers separated the teaching of chemistry
into a theory part and a practical part. They taught theory through chalk
and talk only and drilled students in titrations and identification of
compounds during practical lessons. The Teacher Assessment Scheme (TAS)
was introduced in 1976 hoping to rectify the situation. Now, two modes
of practical examinations are available, It is anticipated that most schools
will use the TAS while the practical examination will be reserved for private
candidates only.
The Advanced Supplementary Chemistry Syllabus is a new syllabus developed
according to the recommendation of the Report of the Working Group on Sixth
Form Education to broaden the Sixth Form curriculum. It is intended as
a terminal chemistry course for Form six students and consists of 9 sections.
The first 8 sections are essentially a trimmed down version of the A-level
syllabus covering the areas atomic structure, bonding and structure, energetics,
rates of chemical reactions, chemical equilibria, the Periodic table, fundamentals
of carbon chemistry, and chemistry of some carbon compounds. Section 9
is a new section added to reflect the aims that students should acquire
an awareness of the social, economical, environmental and technological
implications of chemistry, and, a readiness in becoming responsible citizens
in a changing world. A synopsis of Section 9 is given below:
=========================================================================
Section 9 Chemistry and Society
9.1 Chemistry and the Hong Kong industry
- (a)
- (I) The plastics industry
- (II) The electroplating industry
- (b)
- The economic, environmental and social aspects of industrial chemical
processes
9.2 Chemistry and the environment
(a) Impact of modern living on the environment
(b) Air pollution
(c) Water pollution
(d) Pollution control
9.3 Chemistry and agriculture
(a) Adjustment of soil pH
(b) Fertilizers
(c) Agricultural pesticides and associated environmental problems
9.4 Chemistry and food
(a) Food: proteins, carbohydrates, fats and oils
(b) Food preservation
(c) Food additives
9.5 Chemistry and home hygiene
(a) Soaps
(b) Detergents
(c) Shampoos
(d) Toothpastes
(e) Skin creams and lotions
(f) Drain cleaners and oven cleaners
(g) Glass cleansers and abrasive cleansers
(h) Laundry bleaches
=========================================================================
Top of Page
6. CHEMISTRY LESSONS IN LOCAL
SCHOOLS
A typical school year starts in September and ends in early July. For
secondary schools, the school day typically starts around 8.00 a.m. and
ends at 3.30 p.m. A teacher normally teaches about 28 - 30 periods per
five-day-week, each period is between 35 to 40 minutes. A typical school
day consists of 8 periods per day and there is no school on Saturdays.
In a typical school, there are at least four laboratories, one for each
of the subjects Chemistry, Physics, Biology and Integrated Science. Some
schools may have an extra senior science laboratory. There are normally
two laboratory technicians, one to look after two laboratories, and a number
of laboratory attendants to help cleaning up. At least half of the chemistry
lessons are scheduled in the chemistry laboratory.
A typical class consists of 40 students. Provisions for group work based
on 4 students per group can be easily achieved. A variety of teaching strategies,
e.g. small group work, discussion, debate, role play, hands on experiments
and projects are encouraged. The teaching of communicative skills is emphasized
in the curriculum guide of the new syllabuses. the implementation of these
goals, however, varies greatly from teacher to teacher. The language used
should be English for schools that have opted to be EMI (English medium
of instruction) schools. However, very few classrooms use English as the
medium of instruction as most teachers use a mixed code, i.e. a mixture
of Cantonese (the spoken dialect by most Chinese in Hong Kong) and English.
Despite criticisms from educationist on the undesirable effect of using
mixed code, schools are reluctant to change to the mother-tongue as medium
of instruction because of the perception that the use of English has greater
value in terms of future study and employment opportunities. All tests,
exam and text books are however, in English. There is a small percentage
of schools using Cantonese as the medium of instruction as well as Chinese
text books.
Top of Page
7. STUDENT NUMBERS
In 1995, there were 30,930 candidates sitting for the Certificate of
Education Examination out of a total number of 123,945 candidates taking
the Exam., i.e. about one in four students at F.5 level take chemistry.
There are 9,078 candidates taking the A-level chemistry examination
compared with the total number of 28,787 candidates. About one in three
F.7 students take chemistry. The AS-level chemistry course, however, is
not as popular, only 537 candidates took the exam. in 1995.
Top of Page
8. PUBLIC EXAMINATIONS
The Hong Kong Examinations Authority is the official body responsible
for operating the Hong Kong Certificate of Education Examination and the
Hong Kong Advanced Level and Advanced Supplementary Level Examinations.
It is a self-funding and non-profit making body. It employs full-time staff,
supplemented by a large number of temporary staff at examination times.
There are many serving teachers and academics serving in its many sub-committees.
Some examples of examination questions are given below to indicate the
depth of treatment and the type of skills tested.
At Certificate of Education level, most examination questions are highly
structured.
=========================================================================
Example 1: HKCEE, 1995.
The table below gives some information about five metals.
| Metal |
Abundance in the earth's crust (%) |
Price per Kg ($) |
Relative resistance of corrosion
(1 = least resistant
4 = most resistant) |
Relative strength of metal
( 1 = lowest
3 = highest) |
| Al |
8.1 |
170 |
3 |
1 |
| Cu |
0.0055 |
140 |
3 |
3 |
| Au |
0.0000004 |
1100000 |
4 |
2 |
| Fe |
5.0 |
20 |
1 |
3 |
| Zn |
0.007 |
160 |
2 |
2 |
- (i)
- Although gold has a very low abundance in the earth's crust, gold was
discovered by man a long time ago. Why?
- (ii)
- Which of the metals in the above table is the most suitable to make
pipes for hot water? Explain you answer.
- (iii)
- (1) Aluminium does not corrode easily. Why?
- (2) Aluminium is a principal material for making aircraft but its strength
is relatively low. Suggest how the strength of aluminium can be improved
to make it suitable for making aircraft.
- (iv)
- (1) Based on the information given in the table, suggest ONE factor
that affects the price of a metal.
- (2) Suggest ONE other factor (not indicated in the table)
that can also affect the price of a metal. (9 marks)
=========================================================================
There are, however, questions where candidates have to give paragraph-length
answers. 3 out of the marks for each question are awarded for the effective
communication of knowledge in chemistry.
Example 2: HKCEE 1995
Describe how large crystals of ammonium sulphate can be prepared from an
aqueous solution of ammonia in a school laboratory. ( 9 marks ) |
| Example 3: AS Level Examination, 1995
An example of a structured question:
The ingredients of a brand of apple juice are listed below:
water, cane sugar, concentrated apple juice, sulphur dioxide, citric acid,
vitamin C, sodium citrate, flavourings, stabilizer (E466), natural colour
(E160).
- (a)
- (i) Sulphur dioxide is added to prevent the browning of the apple juice.
Explain the action of sulphur dioxide.
- (ii) Suggest ONE OTHER function of sulphur dioxide in the apple juice.
- (iii) For humans, the acceptable daily intake (ADI) of sulphur dioxide
should not exceed 0.7 mg per kg of body weight. What is the possible menace
of excessive intake of sulphur dioxide? (4 marks)
- (b)
- Sodium citrate is added to regulate the acidity of the apple juice.
Explain the action of sodium citrate. (2 marks)
- (c)
- Why is cane sugar added to the apple juice? ( 1 mark)
|
| Example 4: AS-Level Examination, 1995.
An example of essay type question:
Marks will be allocated as follows:
| chemical knowledge |
9 marks |
| organization and presentation |
6 marks |
You should use equations, diagrams and examples where appropriate.
The examiners are looking for the ability to analyze, to evaluate, and
to express ideas clearly.
Write an essay on the isomerism in organic compounds. (15 marks) |
| Example 5: A-Level Examination questions 1995
Structured question:
- (a)
- (I) Derive an expression for the molar mass M of an ideal gas, in terms
of its density pressure P and absolute temperature T.
- (II) At 98.6 kPa and 300 K, the density of a sample of dry air is 1.146
g dm-3. Assuming that dry air contains only nitrogen and oxygen and behaves
ideally, calculate the composition of the sample.
(Gas constant, R = 8.31 J K-1 mol-1 ) ( 5 marks )
- (b)
- (I) At 298 K, the solubility product of calcium ethanedioate is 2.3
x 10-9 mol2 dm-6.
Predict, with explanation, whether or not calcium ethanedioate will be
precipitated when equal volumes of 2.5 x 10-2 M calcium nitrate(V) and
4.0 x 10 -6 M sodium ethanedioate are mixed together at 298 K.
- (ii) Explain why calcium ethanedioate is more soluble in dilute hydrochloric
acid than in water. ( 4 marks )
|
| Example 6: A-Level Examination 1995
Essay- type questions:
Marks will be allocated approximately as follows:
| chemical knowledge |
50% |
| organization |
30% |
| presentation (including proper use of English) |
20% |
Equations, suitable diagrams and examples are expected where appropriate.
The examiners are looking for the ability to analyze, to evaluate and to
express ideas clearly.
Write an essay on hydrogen bonding. (20 marks ) |
Top of Page
9. CHEMICAL EDUCATION AT THE
UNIVERSITIES
Tertiary education in Hong Kong has expanded dramatically during the
last few years. There was only one University, the University of Hong Kong
in 1963, now there are seven universities funded publicly through the University
Grants Committee. In 1980, only 2.2 per cent of the 17-20 age group could
enter a first-degree programme, now at least 18% of the relevant age group
can obtain a university place in Hong Kong and a significant % study overseas.
Of these seven universities, six offer B. Sc. degree programmes in Pure
and Applied Chemistry and Biochemistry as well as M. Sc. and Ph. D. programmes
in Chemistry, Biochemical Technology and Environmental Science. Degrees
awarded by Hong Kong institutions are recognized around the world.
Candidates who submit HKALE results can apply for admission to universities
through the Joint University Programmes Admissions System (JUPAS). Others
may apply directly to the Universities. JUPAS is a centralized system introduced
in 1990 to prevent students holding places in more than one universities.
Students can choose up to 20 study programmes in any of the 7 universities.
Selection and allocation of places is mainly based on their HKALE results.
Their HKCEE results and confidential academic references are also taken
into consideration. Students may have to sit for aptitude tests and attend
interviews. Each successful applicant receives only one offer, which is
the highest in the preference list submitted by them, then their names
will be deleted from the list, whether they accept the offer or not.
Top of Page
10. CHEMISTRY TEACHERS
Non-graduate teachers used to be trained by the four colleges
of education under the Education Department. From 1994, the Hong Kong Institute
of Education (HKIED) was created from the four colleges, and the Institute
of Language in Education which provides in-service language courses. The
HKIED's main priorities are to upgrade existing non-degree programmes and
to plan the introduction of some degree programmes. After graduation, student
teachers are eligible for the registered teacher status. They can become
Certificate Masters/Mistress (CM) in either primary or secondary schools.
Normally, they can teach up to F.3 in secondary schools. Most Integrated
Science lessons in secondary schools are taught by these CM teachers.
The training of Graduate teachers is mainly provided by the Faculty
of Education of the University of Hong Kong and the Chinese University
of Hong Kong, and recently also by the Baptist College. They offer both
full-time as well as part-time Post-graduate Certificate of Education courses.
The full-time Post-graduate Certificate in Education courses mainly give
pre-service training to graduates. These are one-year-courses and teachers
can also specialize in different major subjects. The two-year-part-time
Post-graduate Certificate in Education courses are mainly for serving teachers
who have not received initial teacher education when they entered the teaching
profession. A graduate with a first degree plus a postgraduate teaching
qualification will be eligible for a registered teacher status.
Chemistry is only taught by graduates. In Hong Kong, one does not have
to be a registered teacher to teach in secondary schools and many graduates
are employed as permitted teachers. A permitted teacher is one who has
an adequate educational background, but no professional teaching qualification.
These teachers may have to teach for two or three years before they are
admitted into the part-time Postgraduate Certificate of Education courses
because of the competition for places.
Top of Page
11. IN-SERVICE TEACHER EDUCATION
PROGRAMS (INSTEP) FOR CHEMISTRY TEACHERS
After teaching for 5 years, teachers are eligible to attend the refresher
training courses (RTC-INSTEP) funded by the Education Department and run
by the Faculty of Education of the University of Hong Kong and the Chinese
University of Hong Kong. These are 100-hour-courses lasting for about a
year. Teachers normally come every alternate Saturdays for about four hours
each. They learn about new developments and trends in their major subject
as well as new developments and issues in curriculum and teaching, and
philosophy of education. They also study skills in the management of departments
to help them to perform their duties as heads of departments. There are
options to enable in depth studies in areas of particular interest to them,
e.g. counselling and guidance, use of the Internet, etc. They are also
expected to attend seminars on current educational issues and tutorials.
Participants also have to complete an independent study. Completion of
the RTC-INSTEP course is a pre-requisite for promotion to Senior Graduate
Master/ Mistress. in 1996, about 250 teachers are undergoing these RTC
courses, of these about 11 are chemistry teachers.
Informal in-service training is also provided by the Inspectorate, the
Curriculum development Institute and the Hong Kong Association for Science
and Mathematics Education in the form of workshops and seminars.
Top of Page
12. Chemical Society and Chemical
Education
The Hong Kong Chemical Society (HKCS) was formed in 1979. It is a founding
member of the Federation of Asian Chemical Societies, and has an exchange
agreement with the Guangdong Provincial Chemical Society. Council members
include those with industrial, public service, and educational affiliations.
The HKCS has always been interested in the promotion of chemical education
in Hong Kong. Among other activities, it organized in 1982, a Symposium
- Exhibition "Chemistry and Industry". It has also sponsored
a series of workshops and lectures/ demonstrations for secondary school
teachers jointly with other organizations like the Education Department
and the Urban Council, e.g. the "Chemistry in Everyday Life".
The Hong Kong Chemistry Olympiad, now in its 7th year, has become an annual
event for tertiary institutions. It is jointly organized by the HKCS, the
Royal Society of Chemistry, the Hong Kong Association for Science and Mathematics
Education and the Education Department to promote chemistry education in
Hong Kong. Six Universities in Hong Kong each entered a team of three chemistry
students. They were given a title shortly before the Olympiad and they
had to give a presentation to a panel of judges in front of an audience.
The topics were given out the day before and were based on a common theme.
The Hong Kong Chemistry Olympiad was extended to secondary schools in
1996. Schools joining the Olympiad each entered a team of three secondary
6 or Secondary 7 students. Each team submitted a written report on a project
and a video which recorded the experiments being done. The six best entries
were selected for oral and video presentation to an audience and a panel
of judges. Examples of projects are studies on apple browning, analyzing
the sucrose content in soft drinks, investigating whether egg white can
be used to remove lead poisoning, determination of chloride ion content
in cheese by using Volhard's method, trying to analyze the chloride content
in different food stuff and to analyze the nitrogen content in food.
The Hong Kong Association for Science and Mathematics Education (HKASME)
has been established in 1966 by a group of dedicated science teachers.
Its membership has grown to 800 and includes many enthusiastic science
teachers and teacher educators. It sets out to provide leadership in science
education for all science teachers in Hong Kong. The major channel of communication
was a bulletin which was expanded into a fully-fledged journal. Vol.1,
Number 1 of The Hong Kong Science Teachers' Journal was first published
in Nov. 1972. The HKASME has done much to promote and support innovations
in science teaching. During the initial implementation of the Integrated
Science curriculum, the Association took a very active role both in providing
in-service training for teachers through seminars and workshops as well
as designing and making home-made apparatus and selling these to schools.
They also organized workshops on the use of such equipment. At the time
when resources in education were limited, the provision of cheap local
home-made apparatus contributed much to the adoption of the curriculum.
Later, as provision for schools improved, the need for such low cost equipment
decreased, also because of the provision of in-service training by the
Education Department, the setting up of the INSTEP (In-service teacher
education program) in the Universities and the offering of training to
teachers by the CDI, the role of the Association becomes less prominent
and there is a decrease in membership in recent years. The Association
is now in urgent need of finding a new direction of development. However,
the HKASME is still active in jointly organizing induction programmes for
beginning teachers, and to initiate innovative projects like the 'Science
Across the Asian Pacific' with other institutions.
The Royal Society of Chemistry also has representatives in Hong Kong
and has been actively involved in promoting chemical education, e.g. in
co-organizing the Chemistry Olympiad. Many members of the Society are practicing
in Hong Kong in education, industry, commerce and other sectors.
Top of Page
13. ENVIRONMENTAL EDUCATION
In the existing school curriculum, there is no independent subject in
the area of environmental education. At primary level, environmental education
is incorporated into the subject General Studies. At secondary level, elements
of environmental education are intended to permeate the curriculum through
subjects like Economic and Public Affairs, Geography, Social Studies, Integrated
Science, Biology, Human Biology, Physics, Chemistry, Religious Education
and Liberal Studies. The Guidelines on Environmental Education in Schools
were prepared by the Curriculum Development Council in 1992 to help schools
to plan and implement environment education. It is intended that 'environmental
education should be implemented in a cross-curricular manner, different
subjects in the school curricular focus on and explore different aspects
of human understanding and experience of the environment, so that students
may get a more holistic perspective of the environment.' The ultimate
aims of environmental Education for schools in Hong Kong are:
'to promote in pupils a lifelong and forward-looking concern for
the environment and to prepare them for making well-informed, justifiable
and practical decisions regarding the conservation of environment so as
to enable them to live as useful and responsible citizens.'
It is expected that environmental education is also implemented through
an informal curriculum by various activities organized within and outside
schools. The Community Youth Club of the Education Department, the Environmental
Campaign Committee, the Urban Council and Regional Council have organized
activities to arouse environmental concern like the annual World Environment
Day, the Environmental Protection Festival, and the 'Keep Hong Kong Clean'
Campaign. Other voluntary organizations like the Conservancy Association,
the Friends of the Earth, Green Power and the World Wide Fund for Nature
Hong Kong also do much to promote awareness for environmental protection
in Hong Kong.
Top of Page
14. DEMAND OF CHEMIST
Although Hong Kong imports about HK$10 billion worth of chemical products
every year, there are few large scale chemical plants in Hong Kong. Although
graduates may find jobs in large companies like the Hong Kong and China
Gas Co. Ltd., Dow Chemical Pacific Ltd., the demand for chemist has not
been great. Chemical engineering graduates usually take up sales executive
posts in trading firms or distributors of industrial chemical and raw materials.
Those with MBAs can apply for positions in marketing, while others can
find work in manufacturing. Those with science degrees in Chemistry can
apply for jobs in the research and development/technical services or in
business development, e.g. banking. New graduates are hired and given on-the-job
training. However, the majority of the graduates with science degrees in
Chemistry go into teaching.
Top of Page
15. CONCLUSION
Chemical education in Hong Kong is undergoing a rapid period of change
since the expansion of tertiary education in Hong Kong during the last
decade. When university places were highly competitive, the ultimate aim
of the chemistry curriculum, for most chemistry teachers, had been to prepare
students to get good grades in the HKCEE and AL examinations so that eventually
they could make it to the universities. The Second International Science
Study (1984-85) showed that "school science education in Hong Kong
is elitist, poor for the majority of students, but attaining very high
academic standards for the few who study science subjects at the secondary
6 and 7 levels" (Holbrook, 1990). With the expansion of tertiary education,
entrance into universities is no longer very competitive, and teachers
have to really re-examine the objectives of a chemistry curriculum. Also,
in Hong Kong, there is the culture of according a high status to science
subjects and schools tend to channel more able students into the science
stream. Therefore, even though there really is not a high demand for chemist,
there is no lacking of students taking chemistry at secondary or senior
secondary level as chemistry is one of the core science subjects. With
Hong Kong being one of the important financial centres of the world, it
is natural to expect that students may have a better prospect studying
economics or computer studies than chemistry. Concern has been expressed
by the Dean/ heads of science departments of the universities that there
are some indications of a swing from science, however, the impact is not
yet felt at the school level. With the expansion of higher education, the
consequence is that one would not be able to expect all students to be
suitable for an elitist education, unless chemistry is perceived to be
interesting, relevant and within their reach, the swing will be inevitable.
The syllabus changes in chemistry were aimed at coping with this. However,
innovations and new ideas are hard to spread to the mass of practicing
teachers. Although teachers coming through the PCED courses of the Faculty
of Education of the Universities are well prepared for their new roles,
every year only about 40 places are available. Only about 10 chemistry
teachers receive refresher training every year, while there are still hundreds
of teachers out there who may have been teaching for over 10 years and
are totally out of touch with the current trends of chemistry education.
Also because of the problem of 1997, many excellent, experienced teachers
are immigrating overseas, thus most chemistry teachers are young, fresh
graduates without any teacher training at all. Although there are one-off
workshops by the Education Departments or CDI on various topics of the
syllabus, articles from Newsletters of the Education Department or the
HKASME that discuss such issues, as well as other innovations like the
'SMILE' project, the school-based curriculum projects etc., that try to
change the practice of teaching and learning in chemistry/science, these
seem to be making little impact. Teaching practices in the classrooms are
still a long way to go in implementing the stated goals and objectives
of the chemistry curriculum in Hong Kong.
References
CDC, 1992, Guidelines on Environmental Education in Schools,
Hong Kong Government Printer.
CDC, 1993, Guide to the Sixth Form Curriculum, Hong Kong Government
Printer.
CDC, 1986, Syllabus for Science (Forms I - III), Hong Kong Government
Press.
CDC, 1994, Syllabus for General Studies (Primary I - VI), Hong Kong
Government Press.
CDC, 1991, Syllabuses for Secondary Schools, Chemistry (Advanced Supplementary
Level), Hong Kong Government Press
CDC, 1991, Syllabuses for Secondary Schools, Chemistry (Secondary 4-5)),
Hong Kong Government Press
CDC, 1995, Syllabuses for Secondary Schools, Chemistry (Advanced Level),
Hong Kong Government Press
Government Secretariat, Education & Manpower Branch, 1994, A guide
to Education and Training in Hong Kong, Hong Kong Government Printer.
Holbrook, J.B., (1990), Education Papers 6, Science Education in Hong
Kong: Achievements and Determinants, Faculty of Education, University
of Hong Kong.
Morris, P.,(1995), The Hong Kong School Curriculum, Development, Issues
and Policies, Hong Kong University Press
Hong Kong Education Dept. Science Subjects Section., 1974, Report of
first year trial of integrated science project, 1973-74, Hong Kong
Government Press.
Hong Kong Education Dept. Science Subjects Section., 1976, Evaluation
of second year trial of integrated science project, 1974-1975, Hong
Kong Government Press.
Chakraborty, D.P.,(1987), 20th Century Chemistry in Asia, Vol. 1, [Hong
Kong, New Zealand and Indian Chapters], G.D. Publisher, Calcutta-700
036.
HKASME, 1972, Hong Kong Science Teachers' Journal, Vol. 1, Number 1.
1 The Education Commission is the highest advisory body on education in
Hong Kong. It advises the government on the development of the education
system in the light of community needs.
Top of Page
Back to Chemical
Education in Ajia Pacific Home Page
![]() A page of a popular Integrated
Science text book
A page of a popular Integrated
Science text book