A. rock salt, B. boric acid, C. copper powder,
D. sodium hydroxide, and E. dry ice,
15.0 g was thrown into 100.0g of water at 20°C and at 40°C. After stirring thoroughly, the solutions were filtered, while keeping the temperature constant. The weights of the liquids thus filtered were measured. The results are given in the table below. Answer the questions. You may assume that the filtration does not affect the amount of water. Answer should be given up to two decimal points.
| --------------------------------------------------- |
Weight of the liquid/g
20°C 40°C
| --------------------------------------------------- |
A Sodium chloride 111.5 115.0
B Boric acid 105.7 108.7
C Copper powder 100.0 100.0
D Sodium hydroxide 115.0 115.0
E Dry ice 100.2 100.1
| --------------------------------------------------- |
(1) There is a limit to the amount of material which can be dissolved in water. Judging from the results at 40°C, answer which of the solutions A to E contains the maximum possible amount of material dissolved. What is the amount which remains on the filter paper ? Follow the example below.
Example: F(5,7)
(2) Which of the solutions filtered are neutral ? Answer with letters A to E.
(3) What is the concentration of the filtered solution ? Answer in percent.
(4) By accident 10g of boric acid and 10g of copper powder were mixed. How much water is needed if the copper is to be recovered by dissolving this mixture in water and filtering the solution at 40°C ?
(5) The construction of the gas burner is as shown in Figure D.1. Suppose the flame is small and strong (i.e., blue flame). You want to get a large and strong (blue) flame. Select the correct actions and arrange them in the right order.
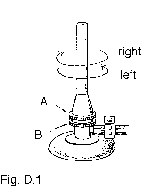 1) Turn the air adjustment screw A right.
1) Turn the air adjustment screw A right.
2) Turn the air adjustment screw A left.
3) Turn the gas adjustment screw B right
4) Turn the gas adjustment screw B left.