Chemical Education in Asia-Pacific
CHEMICAL EDUCATION IN KOREA
Taehee Noh, Inok Han, Kyu Whan Woo, and Sukjin Kang
Department of Chemistry Education, Seoul National University
Shinlim-Dong, Kwanak-Gu, Seoul (151-742), Korea
Back to Chemical Education
in Ajia Pacific Home Page
1. EDUCATIONAL SYSTEM-OVERVIEW
The educational system of Korea is a centralized school system coordinated
through the Ministry of Education. The basic pattern of the school system
is a 6-3-3-4 model; elementary school (ages 6 through 11), junior high
school (ages 12 through 14), high school (ages 15 through 17), and four-year
college and university. Education is compulsory for six years of elementary
school. In rural areas, compulsory education has been extended to junior
high school. In addition to the backbone of the educational system, the
following schools are also available for higher education: 1) six-year
medical and dental colleges; 2) four-year teacher's colleges; 3) two-year
junior colleges, the air and correspondence university, and open universities;
and 4) others like theological colleges and seminaries. The Ministry of
Education controls many aspects by setting criteria and standards regarding
curriculum and degree requirement, qualification of teaching staff, student
quotas, and so forth.
The academic year, which consists of two semesters, begins in March
and ends in February. The minimum number of school days for the completion
of one academic year is established by the Education Law. For primary and
secondary schools, more than 220 school days are required. For higher education
institutes, 32 weeks of school attendance are required. School is in session
from Monday through Saturday (half a school day on Saturday) in primary
and secondary schools.
Korea is relatively homogeneous in terms of ethnicity of its people,
and the language of instruction is invariably Korean.
Top of Page
2. COURSE OF STUDY (GOVERNMENT'S
GUIDELINE)
2.1 Curricula and Textbook System
The Education Law articulates the goals and objectives of education
for each school level. To ensure a standard quality of education, the Education
Law prescribes the curricula for each school level and the criteria for
the development of textbooks and instructional materials. The Ministry
of Education provides the courses of study, which is called "Curriculum".
Since all primary and secondary schools are required to follow the curricula,
they are essentially universal regulations enforcing the teaching objectives
and the contents.
The curricula have been revised on a periodical basis to reflect emerging
needs of changing society and new frontiers of disciplines. Since the first
version established in 1955, it has been revised five times. The sixth
version was announced in 1992. The purpose of the new curricula is to produce
a 1) healthy, 2) independent, 3) creative, and 4) moral person. The principles
for the preparation of the sixth version are: 1) decentralization of decision-making
regarding the curricula; 2) structural diversity of the curricula; 3) adequacy
of the contents in the curricula; and 4) efficiency in the management of
the curricula.
Textbooks are revised and updated according to the new curricula. The
Ministry of Education owns the copyright of the textbooks for elementary
school. The textbooks for science courses in secondary schools are inspected
and selected by the Ministry of Education as well.
Top of Page
2.2. Credit Allotments for Science Courses
Science and social studies are integrated into a course called "Wise
Life" (6 hours a week) for grades 1~2. The "Nature" course
is taught for grades 3~6. The "Science" course is taught in junior
high schools. "General Science" as well as chemistry, physics,
biology, and earth science is taught in high schools. Credit allotments
for science courses at each school level are summarized in Table 1.
Table 1. Credit allotments for science
courses.
| School Level |
Grade Level |
Course |
Credit 1 |
Remark |
| Elementary school |
3
4
5
6 |
Nature |
6
8
8
8 |
compulsory |
| Junior high school |
7
8
9 |
Science |
8
8
8 |
compulsory |
| High school |
10 |
General Science |
8 |
compulsory |
| 11~12 |
Physics I
Chemistry I
Biology I
Earth Science I |
4
4
4
4 |
for nonscience track students |
Physics II
Chemistry II
Biology II
Earth Science II |
8
8
8
8 |
for science track students |
1 One credit consists of one 40 minute class (per week for a semester) in
elementary school, one 45 minute class in junior high school, and one 50
minute class in high school.
Top of Page
2.3 The Science Curriculum of Elementary
School
Science at the elementary school level is taught as an integrated discipline
called "Nature". In the "Nature" course, scientific
attitudes, creative thinking, and decision-making are emphasized. The objectives
of the course are itemized as follows: 1) to acquire rudimentary methods
of inquiring about the nature and utilize them in solving problems; 2)
to understand facts and concepts about natural phenomena and apply them
to explaining natural phenomena; 3) to build interests and curiosity in
natural phenomena and scientific inquiries and to develop scientific attitudes;
and 4) to understand that science influences technological development
and is strongly related to our life.
The contents include not only knowledge such as matter, motion and energy,
life, and earth, but also inquiry activities such as observation, classification,
measuring, communicating, predicting, using models, interpreting data,
experimentation, and so forth. The chemistry contents in the science curriculum
of elementary school are summarized in Table
2.
Table 2. The chemistry contents in the
science curriculum of elementary school.
| Grade |
Unit |
|
Content |
| 3 |
Various matter |
(a) |
Knowledge: color and feel of powders, change of powders when dissolved in
water, change of powders by heat, flow and vaporization of liquids, change
in mixing liquids |
| (b) |
Inquiry activity: observation of color and feel of powders, observation
of the dissolution of powders, observation of the change of powders by heat,
observation of the flow and vaporization of liquids, observation of the
change in mixing liquids, collecting samples of powders and liquids |
4 |
Separation of mixtures |
(a) |
Knowledge: properties of matter, methods of separation |
| (b) |
Inquiry activity: prediction of the components of powders, experiment on
the separation of mixtures, searching for samples of separation in everyday
life |
| Heat and change of objects |
(a) |
Knowledge: relationship between heat and temperature, heat transfer, expansion
of objects by heat, change in states by heat |
| (b) |
Inquiry activity: measurement of temperature, experimentation and data interpretation
on the relationship between heat and temperature, experiment on heat transfer,
observation of expanding objects by heat, observation of the change in states
by heat |
5 |
Dissolution |
(a) |
Knowledge: dissolution of matter, amount of matter that can be dissolved
in solvent, comparison of the weights of matter before and after dissolution |
| (b) |
Inquiry activity: observation of the dissolution of solids in various solvents,
observation of miscible liquids, inference on the dissolution of gas in
liquid, making crystals from solution, comparison of the weights of matter
before and after dissolution, use of a balance |
| Properties of acids and bases |
(a) |
Knowledge: properties of acidic solutions, properties of basic solutions,
reaction of metals in acidic solutions, acids and bases in everyday life |
| (b) |
Inquiry activity: classification of acidic, basic, and neutral solutions,
observation of the characteristics of acidic and basic solutions, making
a neutral solution, observation of the reaction of metals in acidic solutions,
investigation on the use of acids and bases in everyday life |
6 |
Molecules |
(a) |
Knowledge: size of molecules, intermolecular distance, diffusion of gas,
evaporation, sublimation, change in volume accompanying the change in states |
(b) |
Inquiry activity: observation of the dissolution of powders, observation
of evaporation, observation of sublimation, observation of diffusion, inference
on the temperature dependence of the speed of molecular motion, observation
of the change in volume accompanying the change in states, inference on
the intermolecular distance by measuring volume change when two liquids
are mixed |
| Oxygen and carbon dioxide |
(a) |
Knowledge: preparation and properties of oxygen, preparation and properties
of carbon dioxide, combustion |
(b) |
Inquiry activity: collection of gases, observation of the color and smell
of oxygen, investigation on the use of oxygen in daily life, measurement
of the ignition time of a candle in bottles of different volume |
Top of Page
2.4. The science curriculum of junior high
school
Science education at the junior high school level aims at the enrichment
and advancement of the scientific knowledge acquired at the elementary
school level and providing the basis for high school science education.
The course at this level is called "Science". This course is
designed to help students have scientific attitudes, inventive ideas, and
abilities to make reasonable decisions through the development of inquiry
process skills and comprehension of scientific knowledge.
The objectives of the "Science" course are itemized as follows:
1) to acquire scientific inquiry processes and apply them to solving problems
in everyday life; 2) to comprehend basic scientific knowledge and apply
it to explaining natural phenomena; 3) to build interests in science and
science learning and develop attitudes to inquire continuously; and 4)
to understand the influence of science on the development of technology
and the progress of society.
The contents of "Science" are divided into two areas: 1) knowledge
area that includes motion and energy, matter, life, and earth; and 2) inquiry
area that includes observation, measuring, classification, experimentation,
interpreting data, investigation, and discussion. Although concepts in
chemistry, physics, biology, and earth science are taught as a combined
science at this level, the units of the course are clearly divided into
the four disciplines. The chemistry contents in the science curriculum
of junior high school are summarized in Table
3.
Table 3. The chemistry contents in the
science curriculum of junior high school.
| Grade |
Unit |
|
Content |
| 7 |
Properties of matter and separation of mixtures |
(a) |
Knowledge: boiling point, melting point, density, solubility, fractional
distillation, chromatography |
| (b) |
Inquiry activity: measurement of density, measurements of boiling point
and melting point, distillation, use of balance |
| 8 |
Composition of matter |
(a) |
Knowledge: compounds, change of viewpoints on matter, symbols for elements,
equations of simple chemical reactions, flame test, atoms, law of definite
proportion, molecules, Avogadro's law, molecular motion |
| (b) |
Inquiry activity: measurement of mass before and after chemical reactions,
observation of color during flame test, interpretation of the data on relationship
between volume and pressure of gas |
| 9 |
Chemical reactions |
(a) |
Knowledge: electrolytes and non-electrolytes, ions, ionic reactions, detection
of ions, properties of acids and bases, indicators, neutralization reaction,
neutralization of acidic soil, oxidation/reduction reactions |
| (b) |
Inquiry activity: observation of ionic reactions, neutralization titration
of acids and bases, measurement of the acidity of rain, inference on the
reactivity order of metals |
Top of Page
2.5. The Science Curriculum of High School
There are nine courses in the science curriculum of high school: General
Science, Chemistry I, Physics I, Biology I, Earth Science I, Chemistry
II, Physics II, Biology II, and Earth Science II.
"General Science" is a compulsory course for all high school
students. This integrated course is designed to help students develop inquiry
process skills needed to solve everyday problems and understand fundamental
science concepts through inquiry activities. The objectives of the "General
Science" course may be summarized as follows: 1) to understand natural
phenomena and to scientifically solve problems in everyday situations by
practicing scientific inquiry methods; 2) to understand fundamental science
knowledge through inquiry activities and apply it to solving problems creatively;
3) to build interests in natural phenomena and learning science and develop
attitudes to inquire continuously; and 4) to understand the influence of
science on the development of technology and the progress of society.
The contents of "General Science" consist of two areas: 1)
knowledge area such as matter, force, energy, life, earth, and the environment;
and 2) inquiry area such as classification, measuring, predicting, experimentation,
investigation and discussion, and interpreting data. Most chemistry subjects
in "General Science" are included in the unit of "Matter".
The contents in the unit of "Matter" may be divided into two
areas. The knowledge area includes the reactivity of substances, the elements
with similar properties, exothermic and endothermic reactions, and the
factors affecting reaction rate. The inquiry area includes classification
of elements by their properties, measuring the heat of combustion of fuels,
and experiments regarding the effects of the concentration of reactant,
temperature, and catalysts on reaction rates.
The "Chemistry I" course is designed to help nonscience (humanities
and social studies) track students understand some fundamental concepts
and strengthen their problem solving abilities. The objectives of the "Chemistry
I" course may be summarized as follows: 1) to understand fundamental
concepts in chemistry through the inquiries of natural phenomena; 2) to
acquire scientific inquiry methods and apply them to solving everyday problems;
3) to build interests in natural phenomena and chemistry learning and develop
attitudes to inquire continuously; 4) to understand the development of
chemistry knowledge and historical viewpoints on the nature; and 5) to
understand the influence of chemistry on technological development and
human life.
The contents of "Chemistry I" are divided into two areas:
1) knowledge area that includes regularity in the world of matter and compounds
around us; and 2) inquiry area that includes classification, experimentation,
investigation, and discussion. The contents of "Chemistry I"
are summarized in Table 4.
Table 4. The contents of "Chemistry
I".
| Unit |
|
Content |
| Regularity in the world of matter |
(a) |
Knowledge: composition of matter, chemical formula, chemical equations,
particles making up matter, atomic model, atomic weight, molecular weight,
electron configuration, periodic table, ionic bond, covalent bond |
| (b) |
Inquiry activity: comparing properties of elements in a family, experiment
on chemical bonding |
| Compounds around us |
(a) |
Knowledge: water and air, properties and uses of metals, properties of
compounds containing sulfur and nitrogen, simple organic compounds, properties
and uses of macromolecular compounds, medicine and our life |
| (b) |
Inquiry activity: investigation on purification process of water and air,
investigation and discussion on the problems of recycling resources, experiment
on bleaching, investigation on the properties of organic solvents used
in our life, discussion of the problems of plastic wastes |
The "Chemistry II" course is designed to help science (natural
science and engineering) track students to be scientifically literate.
It also serves as a preparatory course by providing fundamental knowledge
and inquiry process skills necessary for majoring in science related fields
in the future. The objectives of the "Chemistry II" course are
summarized as follows: 1) to systematically understand the fundamental
chemistry concepts and apply them to explaining natural phenomena; 2) to
strengthen the abilities to inquire about chemical phenomena scientifically
and apply them to solving problems; 3) to build interests and curiosity
in chemical phenomena and chemistry learning and to develop scientific
attitudes; 4) to strengthen the manipulative skills needed in the inquiries
of chemical phenomena and matter; 5) to understand the development of chemistry
knowledge and historical viewpoints on the nature; and 6) to understand
the influence of chemistry on the development of technology and human life.
The contents of "Chemistry II" consist of two areas: 1) knowledge
area including science of matter, atomic structure and periodic table,
chemical bonding and compounds, states of matter and solution, and chemical
reactions; and 2) inquiry area including observation, classification, measuring,
experimentation, predicting, and investigation. The contents of "Chemistry
II" are summarized in Table 5.
Table 5. The contents of "Chemistry
II".
| Unit |
|
Contents |
| Science of matter |
(a) |
Knowledge: atoms, molecules, ions, chemical formula, atomic weight, molecular
weight, mole, chemical equations |
| (b) |
Inquiry activity: experiment on the quantitative relationship |
| Atomic structure and periodic table |
(a) |
Knowledge: components of atom, atomic model and electron configuration,
periodic table, periodic properties of elements, elements of alkali family,
the halogens, transition elements |
| (b) |
Inquiry activity: experiment on the properties of elements in a family,
prediction of periodicity with the periodic table |
| Chemical bonding and compounds |
(a) |
Knowledge: ionic bond, covalent bond, metallic bond, bond polarity, molecular
geometry, organic compounds |
| (b) |
Inquiry activity: observation of properties of ionic and covalent compounds,
experiment on properties and preparation of organic compounds |
| States of gases, matter and solution |
(a) |
Knowledge: ideal gas equation, diffusion, pressure of a gas mixture, liquid
and solid, solution |
| (b) |
Inquiry activity: measurement of molecular weight of gas, experiment on
the properties of solution, preparation of standard solution |
| Chemical reaction |
(a) |
Knowledge: heat of reaction, reaction rate, chemical equilibrium, acids
and bases, neutralization titration and pH, salts, oxidation number, chemical
cell, electrolysis |
| (b) |
Inquiry activity: observation of the shift in equilibrium, experiment on
the neutralization titration of acids and bases, measurement of electromotive
force of cell |
Top of Page
3. Comparison among Textbooks
In Korea, the contents on atoms and atomic structure are not introduced
in elementary school science courses. They first appear in the first unit
of "Science" for 8th graders. The concept of the atom is introduced
after the subunit of compounds and elements. The contents of the atomic
model and compounds in the "Science" textbook published by Kyo-Hak
publishing company, for example, can be summarized as follows: 1) Dalton's
atomic model; 2) historical changes of atomic models; 3) atomic model and
the law of conservation of mass; 4) the law of definite proportions; and
5) the law of multiple proportions. A sample page of the textbook on atoms
and atomic structure is shown in Figure 1.
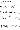 Figure 1
Figure 1
The contents covered in this page are summarized as follows.
According to Dalton's atomic model, atoms are not fundamentally changed
in any chemical reactions. The number and the mass of atoms as well as
the type of atoms remain unchanged, but their arrangements do change. Therefore,
many materials may be used as atomic models to explain atoms. Examples
are in Figure I-29. A magnesium atom may be represented by a small ball,
a bolt, or a paper clip, and an oxygen atom may be represented by a big
ball, a nut, or a clothespin. In explaining chemical bonding of atoms,
it is convenient if the bolt and the nut are represented by the symbols
B and N respectively. The example (b) in Figure I-29 can be symbolized
as follows: B + N ----> BN. When B and N are combined to form the compound
BN, compare the total weight of B and N and the total weight of compound
BN. What will happen if real substances undergo chemical reactions? For
examples, precipitates or gases are produced in some chemical reactions.
Let's find the changes in mass in several chemical reactions.
In high school chemistry textbooks for science track students, atomic
structure is introduced in the third unit "Atomic Structure and Periodic
Table". For example, the "Chemistry II' textbook by Keum-Sung
publishing company summarizes the concepts of atomic structure as follows:
1) electrons and their properties; 2) atomic nucleus model; 3) Bohr's atomic
model; 4) atomic orbitals; 5) three-dimensional atomic model; 6) energy
levels in an atomic orbital; and 7) the arrangement of electrons in an
atom. A sample page of the textbook on atoms and atomic structure is shown
in Figure 2.
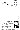 Figure 2
Figure 2
Figure 1. A sample page on atoms and atomic
structure in a junior high school science textbook.
Figure 2. A sample page on atoms and atomic
structure in a high school science textbook.
The contents of this page are summarized as follows.
Based on the results of a study of the electron using a cathode-ray
tube, Dalton's atomic model that the atom cannot be further divided was
questioned. However, no valid model for the atomic structure was proposed
before 1911. In 1911, Rutherford obtained the following results from experiments
on the scattering of alpha particles. First, most alpha particles pass
through a metal foil with a thickness of 4x10-5 cm undeflected. This means
that most of the volume of an atom is relatively empty space. Second, one
out of approximately 2x104 alpha particles which are positively charged
and relatively massive undergoes deflection at an angle of more than 90
degrees. This means that each atom has a particle which is much heavier
and positively charged in a small space. The particle is the atomic nucleus.
Top of Page