4.1. Representative experiments
Two experiments in one of the textbooks for nonscience track high school
students are presented. The first experiment is to introduce the particulate
nature of matter. The second experiment is to introduce the polarity of
covalent compounds.
Experiment 1: How thin and how large of a film of soup solution can
we make?
Materials: wire, thread, straw, aluminum dish, soap solution, beaker,
pin
Experimental procedure:
1. Shape the wire into a loop.
2. Dip the wire loop into the soap solution, take the loop out of the
solution, and hold it vertically as shown.
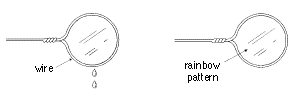
3. Count the dripping drops until the soap film breaks down.
4. Repeat steps 2~3 ten times.
-> How many drops of the soap solution fall from the soap film?
5. Repeat step 2, and observe the change of rainbow patterns until the
soap film breaks down.
-> When do you think the soap film is at its thinnest state?
6. Make a square shape using a straw and thread as shown. By extending
the length of the thread, make the largest soap film.
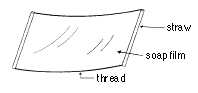
-> What is the width of the largest soap film that you made?
Discussion:
1. Compare the results of the steps 2 and 3. Is the size of soap film
related to the quantity of the soap solution from which it is made?
2. Think about the ways to show that your soap film is thinner than
that of your classmates.
3. Compare the sizes of the soap films we made. Is the size of the soap
film that we can make limited to a certain extent?
Experiment 2: The polarity of covalent compounds
Materials: 500 mL Erlenmeyer flask, rubber stopper, water, toluene,
carbon tetrachloride, thick cardboard, pencil, punch
Experimental procedure:
1. Place 150 mL of water and 150 mL of carbon tetrachloride in a 500
mL Erlenmeyer flask.
2. Blacken one side of the thick cardboard with a pencil.
3. Punch out the pencil-blackened cardboard to make many paper disks.
4. Place the paper disks in the flask, stopper the flask, and shake
the flask.
-> Observe how the paper disks are oriented as the layers form.
5. Place paper disks which have different colors on both sides in the
flask of the step 1, stopper the flask, and shake the flask.
-> Compare the results with those of the pencil-blackened paper disk.
6. Repeat the steps 1~5 with water and toluene.
Discussion:
1. Compare the polarities of the pencil-blackened side and the other
side.
2. Why is the orientation of the pencil-blackened paper disks in water
and carbon tetrachloride different from that in water and toluene?
Top of Page
Since there are several types of high schools in Korea, it is sensible
to describe high schools before explaining the entrance examination system
for higher education.
5.1. High Schools
High schools are mainly divided into academic high schools and vocational
high schools. Most students in academic high schools concentrate on the
preparation for the application for higher education. Therefore, a major
motive to do well in academic high schools is college entrance. Eleventh
graders are divided into two groups: 1) science (natural science and engineering)
track; and 2) nonscience (humanities and social studies) track. Each group
takes relevant courses. Vocational high schools aim at providing advanced
general education as well as vocational training in agriculture, technology,
commerce, and fishery. The comprehensive high schools, which feature the
combination of academic and vocational courses, are also available. Since
1974, the entrance to high schools in equalized areas is based on the results
of a collective examination, while high schools in non-equalized areas
require admission tests conducted by individual schools. In equalized areas,
allocation is made in the first round to vocational high schools, and in
the second round to academic high schools. Those who had wanted to apply
for academic high schools and those who failed to obtain the admission
from vocational high schools take the collective examination of the local
area. The examination usually consists of a written test and a physical
fitness test. The candidates who have passed the examination are assigned
by lottery to schools within their respective school districts. In 1993,
94.6 percent of junior high school graduates went to high schools.
In addition, foreign language, science, art, and athletic high schools
have been founded to identify the gifted at an early age and develop their
potentials. Since 1984, the science high schools have been established
with the nation's paramount interest in advancing scientific and technological
development. There are 13 science high schools across the nation. To be
qualified for entrance into a science high school, the candidates should
be within the top three percent. The entrance examination consists of the
collective examination in the local area and the entrance examination administered
by the school. Those who have completed two years of courses in science
high school are allowed to enter the Science and Technology University.
Top of Page
5.2 Entrance For Universities and Junior
Colleges
The entrance examination system for colleges and universities has become
an important public issue which captures the heart and mind of most students
and their parents. Before 1968, colleges and universities selected their
students on the basis of the scores on the entrance examinations conducted
by individual institutes. From 1969 to 1979, the applicants who passed
the preliminary examination for college entrance were allowed to take the
main entrance examinations conducted by individual institutes. In 1980,
the preliminary examination for college entrance was replaced by the scholastic
achievement test for the college entrance. The scholastic achievement test
score and the norm-referenced high school achievement score were important
determinants for college entrance. Since 1985, essays scores had also been
counted.
To reduce the problems of examination-bound education of high school,
a new entrance examination system was announced in April 1991, which was
effective as of 1994. In the new system, high school achievement (norm-referenced)
score should be counted at least 40 percent. In addition to the scholastic
achievement test, individual institutes are free to administer the main
entrance examinations. They are also free to determine the relative weights
among student achievement, the entrance examination score, and other determinants.
Candidates are also allowed to apply for a special entrance without taking
the institute-administered examinations. In this case, his or her score
of the scholastic achievement test and high school achievement score are
the major determinants of eligibility. Dates of examination are set by
the Ministry of Education in different groups so that each university selects
at its convenience. Students are allowed to apply for many universities,
if they offer examinations on different dates.
The number of junior colleges, which established in response to increasing
demand for technical personnel in 1979 , has grown to 135 with an enrollment
of 506,806. The basic requirement for entrance is met by graduation from
high school. From 1994, the entrance to junior colleges is determined on
the basis of school achievement, scholastic achievement test, main entrance
examination, interview, and aptitude test. However, most schools have used
school achievement and scholastic achievement test. About 40 percent of
the freshmen quota is reserved for the graduates of vocational high schools
in the same fields, craftsmen qualified by the National Certification System,
and workers meeting a specified amount of industrial experiences.
In 1994, 64% of academic high school graduates and 15.1% of vocational
high school graduates went to higher education institutes (Table 6). The rate for female vocational high
school graduates is increasing from 37.7% in 1970 to 45.5% in 1994. Since
it is very competitive to enter prestigious universities and colleges,
many high school graduates spend one or two year(s) to prepare for entrance
examinations in private informal schools. Many secondary students also
take lessons in academic subjects important in the entrance examinations
(mainly Korean, English, mathematics, and science) after school. These
lessons are offered by tutors and/or informal schools which are predominantly
privately operated. Secondary school teachers and professors are not allowed
to offer the lessons. Since the annual education cost for private lessons
is very high, it has become a social problem.
Table 6. Entrance rates (%) of graduates
from high school to higher education.
| Year |
Entrance Rates (female students) |
|
Total |
Academic high school |
Vocational high school |
|
|
Higher education |
Higher education |
| 1970 |
26.9(37.7) |
41.9(41.2) |
9.6(18.7) |
| 1975 |
25.8(37.3) |
41.5(39.1) |
8.8(28.2) |
| 1980 |
23.7(39.3) |
34.0(44.3) |
10.1(16.8) |
| 1985 |
36.4(42.2) |
53.8(43.7) |
13.3(34.6) |
| 1990 |
33.2(45.4) |
47.1(46.0) |
8.3(39.7) |
| 1994 |
45.3(45.5) |
64.0(45.6) |
15.1(45.0) |
Top of Page
5.3. The Scholastic Achievement Test
Since the scholastic achievement test organized by the government is
an important factor in college entrance, which partially determines the
quality of the college to which the student may be admitted as well as
whether the student will continue with education, all applicants to colleges
and universities should take the test. Since the freshmen quota is limited
compared to the number of the applicants as shown in Table
7, the test is very competitive.
Table 7. The number of freshmen quota
and applicants who took the first round scholastic achievement test for
colleges and universities.
| Year |
Freshmen Quota |
Applicants |
| 1989 |
139,856 |
597,099 |
| 1990 |
143,414 |
655,414 |
| 1991 |
146,346 |
662,469 |
| 1992 |
156,111 |
639,485 |
| 1993 |
164,250 |
598,007 |
Since 1994, the nature of the scholastic achievement test has been changed.
The test was also administered twice in 1994. About seven hundred fifty
thousands took the test each time. The total score of the new scholastic
achievement test is 200 points. The test consists of four subtests in the
areas of linguistic potential (60 points), mathematics (40 points), inquiry
(60 points), and lucidity of foreign language (40 points), all focusing
on high mental processing skills and analytic ability. The subtest regarding
inquiry consists of science inquiry and social inquiry. Test items in science
inquiry are in a multiple choice format. In order to increase the discriminating
power, the test items have different weights (0.8, 1.0 and 1.2 points).
The test items have been prepared by professors and checked by high school
teachers. The test items in science inquiry are generally prepared with
a two-way chart - the contents and the inquiry processes. The contents
consist of chemistry, physics, biology, and earth science. The inquiry
processes include identifying problems and formulating hypotheses, designing
and conducting experiments, analyzing and interpreting data, and concluding
and evaluating. Some items are interdisciplinary. A sample item in chemistry
is illustrated in Figure 3.
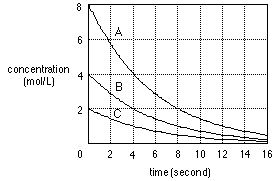
Figure 3. A sample chemistry item in the scholastic achievement test.
The following graph illustrates the changes in reactant concentration
over time when initial concentrations are different in a chemical reaction.
The half-life is the time required for the concentration of a reactant
to decrease to one-half.
Select the one that includes all correct explanations about the graph
in the box below.
| a. The unit of the reaction rate is mol/L*sec.
b. The initial reaction rate is independent of the concentration.
c. The rate constants are different in the cases of A, B, and C.
d. The half-life is 4 seconds.
1) a, b 2) a, c 3) a, d 4) a, b, d 5) b, c, d
|


